- Product Details
Keywords
- Atosiban supplier
- Atosiban
- high quality Atosiban
Quick Details
- ProName: Atosiban manufacturer
- CasNo: 90779-69-4
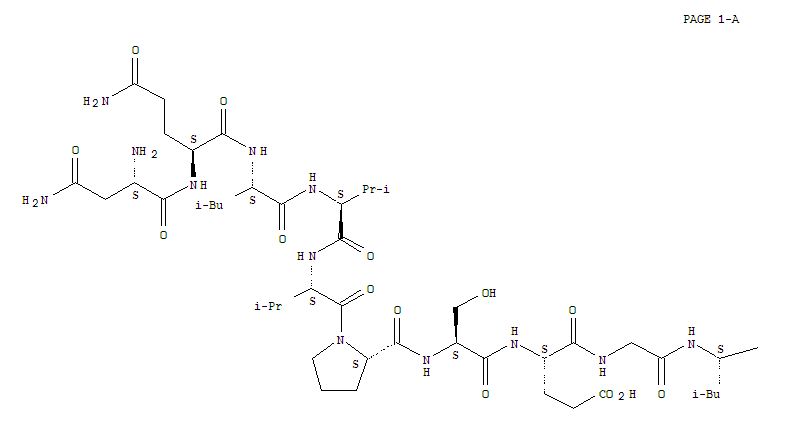
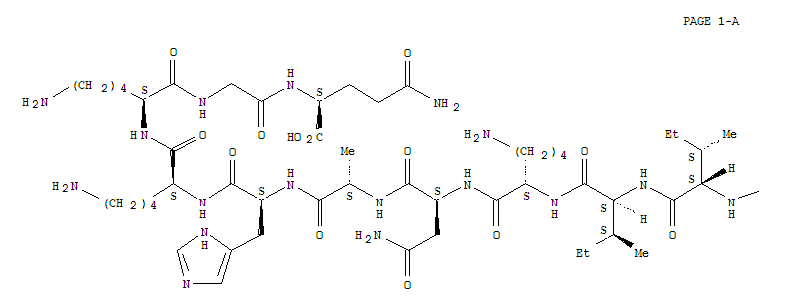
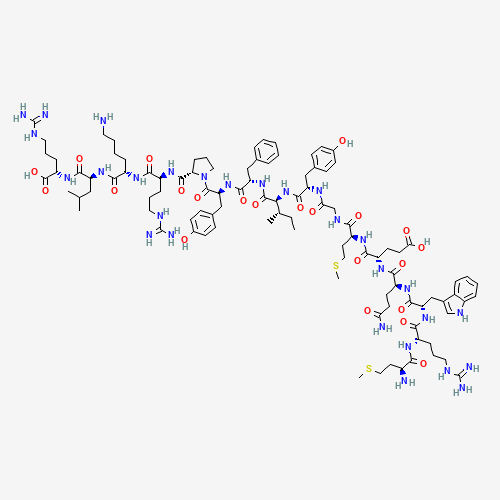
- Molecular Formula: C45H71N11O14S2
- Appearance: White powder
- Application: pharmaceutical
- DeliveryTime: 15days to 1month
- PackAge: According client's requirements
- Port: shanghai
- ProductionCapacity: 1000 Gram/Month
- Purity: 0.98
- Storage: keep sealed and keep from direct light
- Transportation: According client's requirements
- LimitNum: 1 Gram
- Moisture Content: NML 7%
- Impurity: NML2.0%
- peptide content: NLT80%
Superiority
Our Advantages:
1.Product Capacity: eptidego has produced thousands of peptides ranging in quantity from milligrams to multiple kilograms with purities up to 99.0%.
2. Samples free trial: our company welcome samples to test our quality then make regular orders.
3. Good Service: the company have 24 hours online service and feedback to our clients ontime
4. Quality guarantee: We have strict quality control system before our delivery and refunds or re-deliver if any quality problems.
5. Fast Delivery and safe shipping: The compnay process orders in time and have much experienced in internation shipping, always keep goods safe shipping and fast delivery.

Details
Atosiban (trade names Tractocile, Antocin, atosiban SUN) is an inhibitor of the hormonesoxytocinand vasopressin. It is used as an intravenous medication as a labour repressant (tocolytic) to haltpremature labor. Although initial studies suggested it could be used as a nasal spray and hence would not require hospital admission, it is not used in that form. It was developed by Ferring PharmaceuticalsinSweden and first reported in the literature in 1985.Originally marketed by Ferring Pharmaceuticals, it is licensed in proprietary and generic forms for the delay of imminent pre-term birth in pregnant adult women
|
Contents of testing |
Specifications |
Results |
||
|
Appearance |
White or almost white crystalline powder |
Complies |
||
|
Identity by HPLC |
The reaction is same with the reference substance |
Complies |
||
|
Specify Optical Rotation |
-45.0~-55.0° [a]20 D(c=1, 1%HAc) |
-51.0 |
||
|
Amino Acid Composition |
±20% of theoretical |
Complies |
||
|
Purity (HPLC) |
≥ 98.0% |
98.8% |
||
|
Related Substance(By HPLC) |
Total Impurities(%)≤ 2.0% Largest Single Impurity(%)≤ 1.0% |
1.2% 0.6 % |
||
|
Acetate Content(By HPLC) |
≤15% |
6.7% |
||
|
Water Content(By HPLC) |
≤5.0% |
2.8% |
||
|
Peptide Content(N%) |
≥80.0% |
88.5% |
||
|
Bacterial Endotoxins |
≤5IU/mg |
Complies |
||
|
Assay(By anhydrous, acetic acid-free) |
95% ~103% |
98.0% |
||